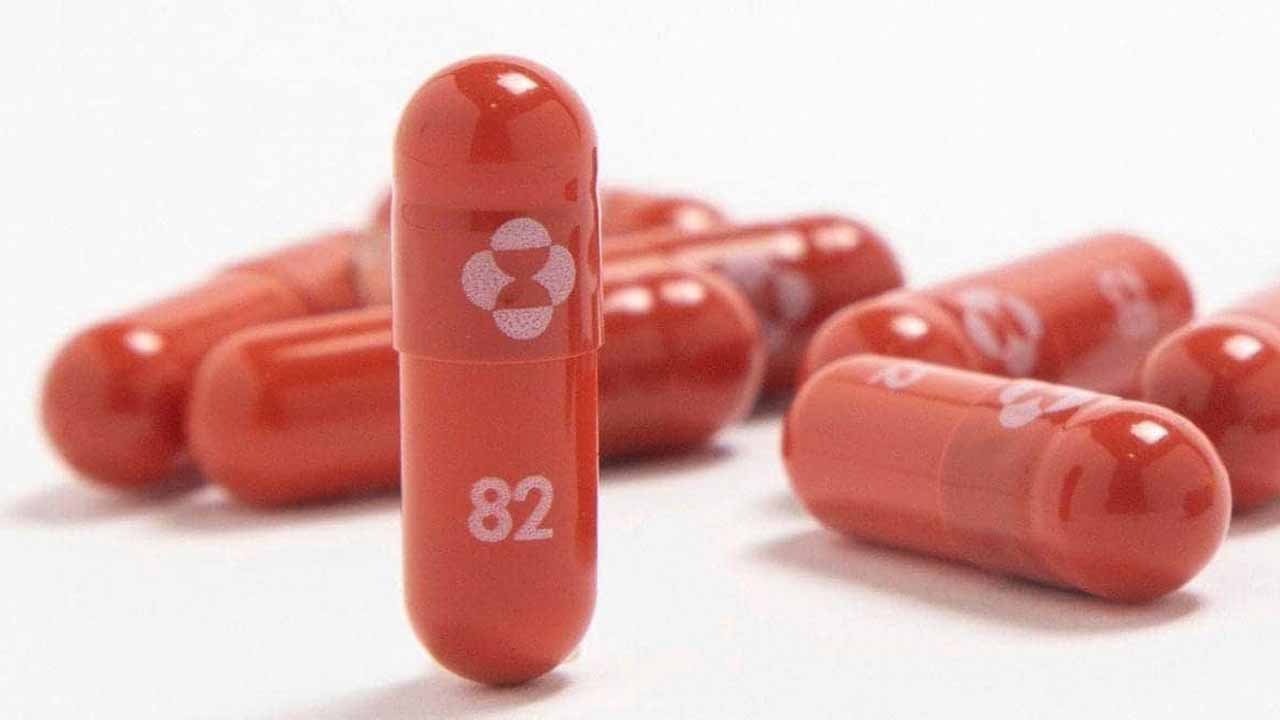
With a fresh spike in Covid-19 cases across the nation, the first consignment of anti-viral drug Molnupiravir, which recently received the nod from regulators in India, was distributed among some top private hospitals in Mumbai, raising hope in the fight against the virus and its new variant Omicron. The BMC (Brihanmumbai Municipal Corporation) has floated a tender to procure the first anti-viral Covid-19 pill Molnupiravir that got approval from the Indian drug regulator on December 28. The drug is already being prescribed in the private sector since it became available last Friday.
Thirteen Indian pharmaceutical companies, including Torrent, Cipla, Sun Pharma, Dr Reddy’s, Natco, Mylan, Hetero and Optimus will be manufacturing Molnupiravir, which is being developed by US-based biotechnology company Ridgeback Biotherapeutics in collaboration with US pharma giant Merck.
How Molnupiravir Works?
Molnupiravir works by disrupting the virus’s reproduction. Once the virus gets inside the body’s cells, it replicates its genome, which is made not of DNA but RNA (ribonucleic acid). These replicated genomes are then formed into complete virus particles which burst out of the cell and continue to spread around the body.
However, the molecules of Molnupiravir are absorbed by virus-infected cells, where they are converted into a defective version of the building blocks of RNA. So, when the virus tries to replicate, the resulting virus particles have defective genetic material and can no longer reproduce. This means that the viral load should remain low, which reduces the risk of serious disease.
Since Molnupiravir targets the RNA that SARS-CoV-2 uses as its building blocks, it should be equally effective against all coronavirus variants.